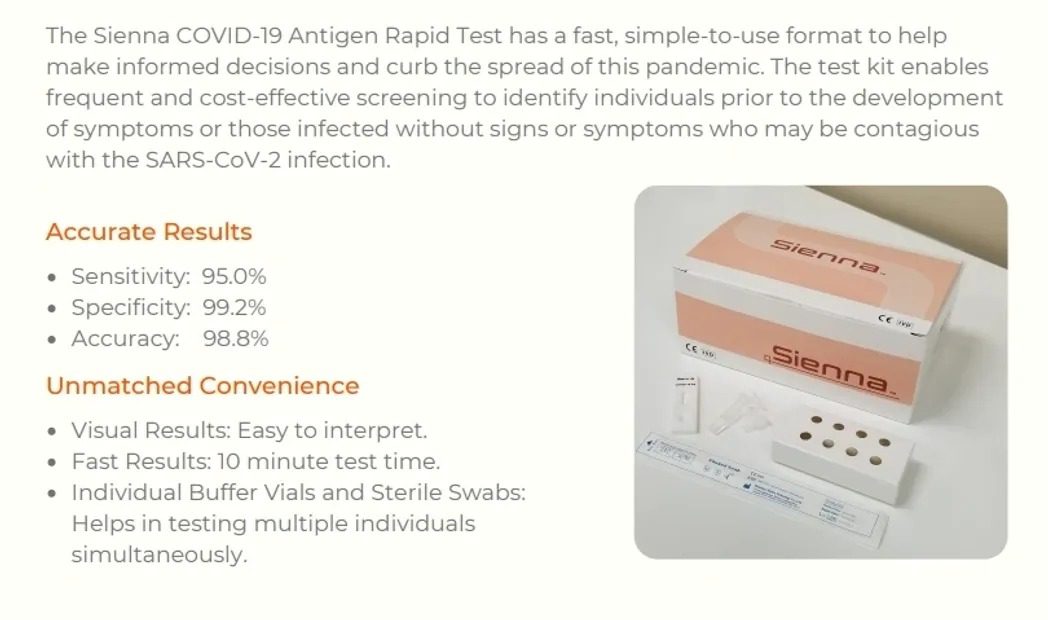
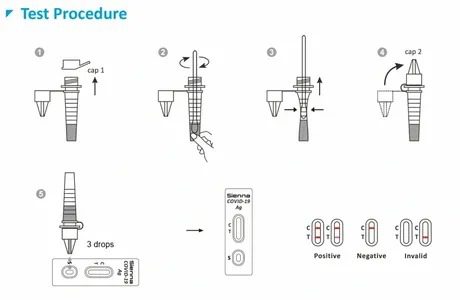
CERTIFICATIONS AND RECOGNITIONS










Sienna Antigen
We place great value on trust and integrity, and we are committed to pursuing continual innovation and development in everything that we do.
We manufacture and market high-quality antigen test kits for the medical industry.
Analytical Performances of the Point-of-care for Sienna™
Published By: International Journal of Infectious Diseases
Analytical Performances of the Point-of-care Sienna™ Covid-19 Antigen Rapid Test
View Full Document: https://doi.org/10.1016/j.ijid.2021.03.051
VOLUME 106, P8-12, MAY 01, 2021
The SIENNA™ Ag-RDT presents excellent analytical performances for viral loads ≤33 Ct, classically co
Objectives
We herein assessed the analytical performances of the antigen-rapid diagnostic test (Ag-RDT) SIENNA™ COVID-19 Antigen Rapid Test Cassette (Nasopharyngeal Swab) (Salofa Oy, Salo, Finland), targeting the SARS-CoV-2 N nucleocapsid protein, for the diagnosis of COVID-19 in hospitalized patients with suspected SARS-CoV-2 infection, by reference to real-time RT-PCR (rRT-PCR).
Methods
Nasopharyngeal swabs were collected from patients with COVID-19-like illness during the second epidemic wave in Paris, France, among which 100 and 50 were positive and negative for SARS-CoV-2 RNA, respectively.
Results
Overall, the Ag-RDT showed high sensitivity, specificity, positive and negative predictive values of 90.0%, 100.0%, 100.0% and 98.1%, respectively, as well as high or almost perfect agreement (93.3%), reliability assessed by Cohen’s κ coefficient (0.86), and accuracy assessed by Youden’s J index (90%) to detect SARS-CoV-2. The analytical performances of the Ag-RDT remained high in the event of significant viral excretion (i.e., N gene Ct values ≤33 by reference rtRT-PCR), while the sensitivity of the Ag-RDT dropped to 69.6% with low or very low viral shedding (Ct > 33).
Conclusions
The SIENNA™ Ag-RDT presents excellent analytical performances for viral loads ≤33 Ct, classically corresponding to situations of symptomatic COVID-19 and/or proven contagiousness.
The coronavirus disease 2019 (COVID-19) pandemic continues to spread across the world. Hence, there is an urgent need for rapid, simple, and accurate tests to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. While currently recommended nucleic acid amplification tests (NAAT), such as real-time reverse transcription polymerase chain reaction (rtRT-PCR) assays, remain the gold standard cornerstone for the diagnosis of SARS-CoV-2 infection (Smithgall et al., 2020, Rai et al., 2021), immunological methods can also be used to detect viral antigens (Dinnes et al., 2020, Li and Li, 2020, Rai et al., 2021). Indeed, performing rtRT-PCR is expensive, time consuming, and requires special equipment and qualified operators. Faster, cheaper, and easier to use alternative tools could be represented by novel point-of-care antigen-detecting rapid diagnostic tests (Ag-RDT) (Dinnes et al., 2020). Ag-RDT relies on direct detection of SARS-CoV-2 viral proteins produced by replicating virus in nasal swabs and other respiratory secretions, often the virus N nucleocapsid protein, preferred because of its relative abundance and conserved structure, or other viral proteins such as the spike protein (Li and Li, 2020). Most Ag-RDTs use sandwich catching by anti-SARS-CoV-2 monoclonal antibodies to detect viral antigens in the simple-to-use lateral flow immunoassay format allowing results in <30 min. Around 150 Ag-RDTs for SARS-CoV-2 infection are now commercially available or in development (Finddx, 2021). However, there is significant variability reported with respect to their diagnostic performances and a lack of external validation for many of the available tests, which still require clinical validation (Dinnes et al., 2020, Mattiuzzi et al., 2020, Favresse et al., 2021, Fitzpatrick et al., 2021, Schildgen et al., 2021).The aim of our study was to evaluate the Ag-RDT SIENNA™ COVID-19 Antigen Rapid Test Cassette (Nasopharyngeal Swab) (Salofa Oy, Salo, Finland; manufactured under license of T&D Diagnostics Canada Pvt. Ltd., Halifax, Canada), for the diagnosis of COVID-19 in hospitalized patients with suspect SARS-CoV-2 infection during the second wave of the COVID-19 epidemic in Paris, France. The results of this test were compared with qualitative and quantitative results obtained in parallel using rtRT-PCR as reference test.
FDA EUA AND POC (Point of Care) Authorized

Sienna Antibody Test
FDA EUA and POC (Point Of Care) authorized Sienna-Clarity COVID-19 IgG/IgM antibody test kit for SARS-CoV-2.
The Sienna rapid test kit delivers a result in ten minutes and meets the FDA performance thresholds for serology test kits. The Sienna test kit was independently validated at the Frederick National Laboratory for Cancer Research, a federally funded research and development center sponsored by the National Cancer Institute.
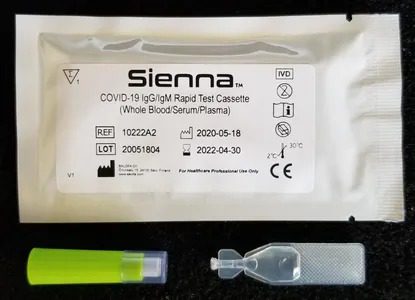
Individual Buffer
Each test comes with it's own buffer dispenser reducing the risk of in field contamination.

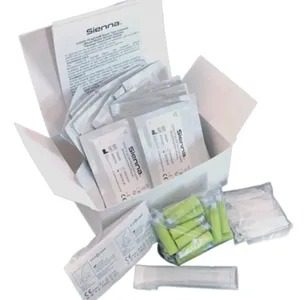
Complete Test
We have everything, even the alcohol pad. Please check the package insert for details.